Male’, Maldives — The Health Protection Agency (HPA) informed that all the vaccines used in Maldives will be approved and registered with the Maldives Food And Drug Authority (MFDA) before it is administered to the public.
HPA stated that all the vaccines used in Maldives will meet the safety requirements given by the World Health Organization and will be assessed to ensure that the standards of safeness and effectiveness is met.
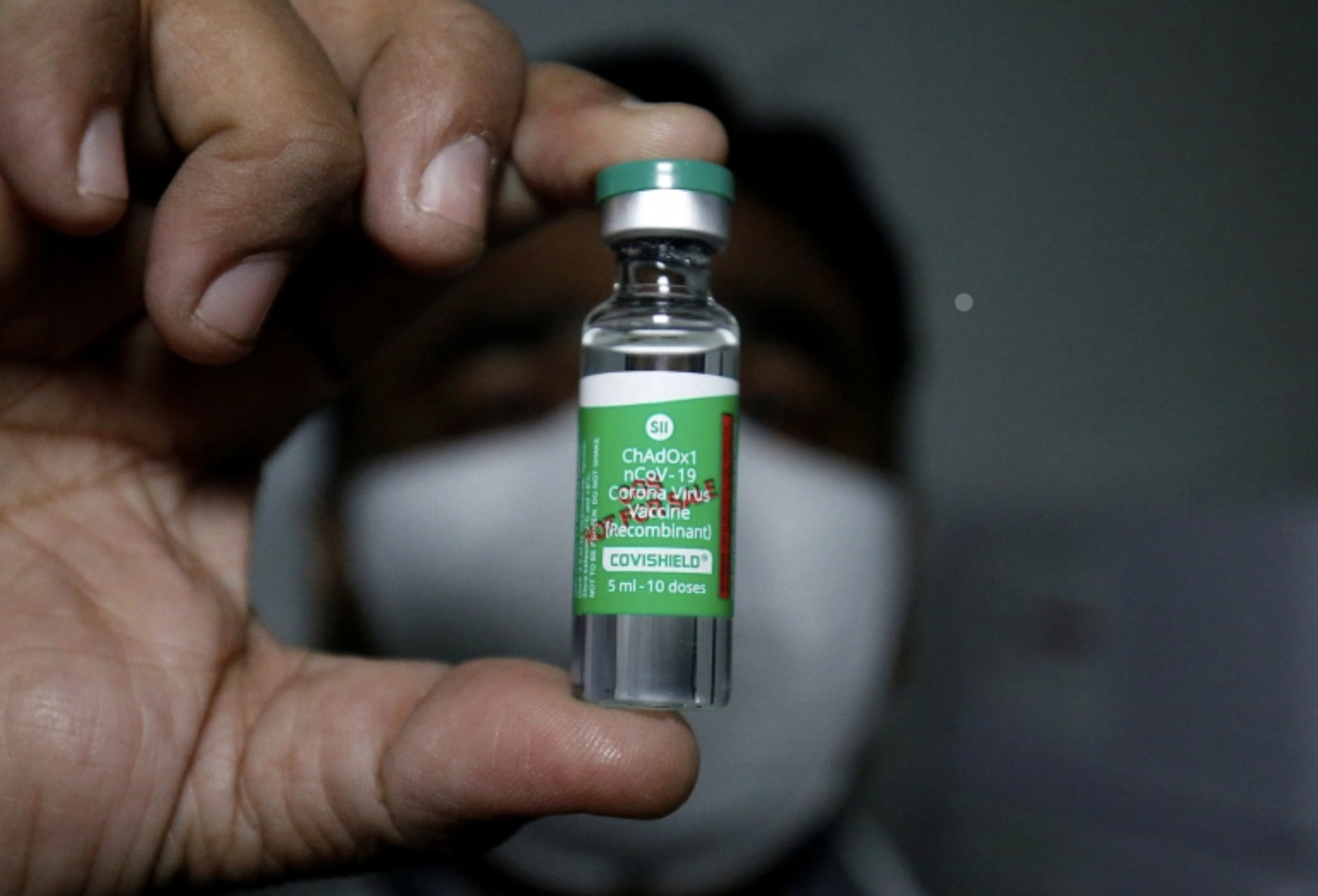
HPA stressed that the Oxford-AstraZeneca’s Covishield vaccine currently in Maldives will only be be administered to the residents of the country after getting approved by the Food and Drug Authority of the Maldives, following thorough investigations into the safety of the jab.
Maldives received its first Covid-19 vaccine shipment yesterday. The first shipment consisted of 100,000 doses of the Covishield vaccine which is expected to be administered to 50,000 individuals – health workers and frontliners are given priority.
The Covishield vaccine brought to Maldives is manufactured by one of the largest vaccine manufacturers in the world, Serum Institute of India Pvt. Ltd. HPA informed that this vaccine has the same formulation that was co-developed by Oxford University and AstraZeneca to combat COVID-19.
HPA informed that similarly as other vaccines, the development process of the Covishield vaccine was also done at a fast phase, however, the development process of the vaccine had ensured safety. The Covishield vaccine has also gone through three testing trials which included of the same number of participants as traditional ones.
The Covishield vaccine is currently going through its n Phase III (large scale) clinical trials, HPA ensured that all the vaccines that will be used in Maldives will be assessed and approved by MFDA before administration.
While the Covishield vaccine is currently being tested by MFDA, the country is currently working on registering those who want to get vaccinated against the novel coronavirus, for which a dedicated online portal, my.health.mv has been launched.
As per the current priority categories, the vaccine will be first administered to health and social workers, followed by individuals above the age of 50. After this, individuals between the age of 18-50 who fall in the high risk category will also be receiving the shot. Next in line to receive the shot would be teachers, police and tourism industry personnel.