Male’, Maldives – Ever since the novel coronavirus broke out in China, which eventually snowballed into a global pandemic, crippling economies and overwhelming health care systems all the while claiming thousands of lives each day, the one thing anyone could have wished for would have been a way out of of this. A day we get to take back our lives, and take a fresh breath out in the open without he fear of getting infected.
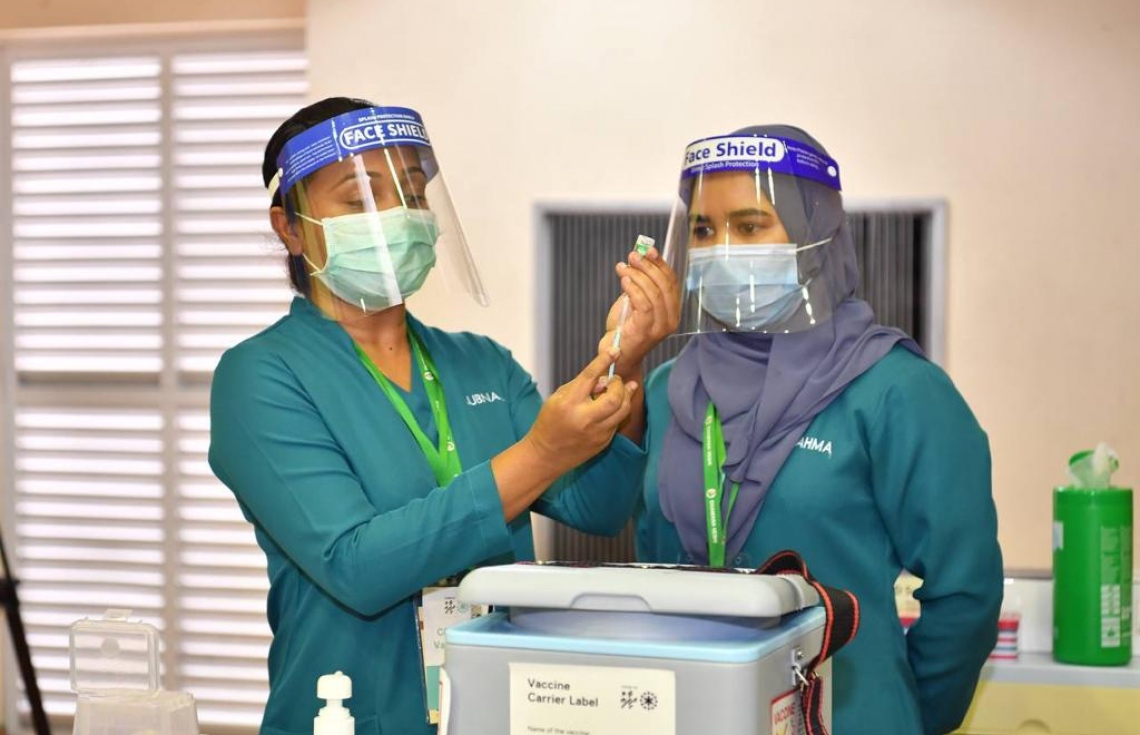
Luckily for the Maldives, the beginning of that day has dawned. Today, Maldives launched the ‘Covid-19 Dhifaau’ campaign, which, according to the President of the Maldives, will see the entire Maldivian population inoculated against the coronavirus.
What is Covid-19 Dhifaau campaign?
The Covid-19 dhifaau campaign is a nation wide vaccination program against Covid-19, which over time, will allow the residents of Maldives to be free from the Covid-19 virus. The program was launched today, by President Ibrahim Mohamed Solih, where several top government officials, as well as health care workers across the country, specifically in the northern, central and southern most areas received the anti Covid-19 jab,
The program is likely to go on for at least the next six months, covering the entire country, allowing immunization against the virus, that has already claimed 52 lives in the Maldives.
Which vaccine are we getting?
The program started today with 100,000 doses of the Covid-19 vaccine produced by AstraZeneca Pharmaceuticals, in collaboration with Oxford University, and manufactured by the Serum Institute of India. These vaccines were donated to the Maldives by India, which saw several other neighboring countries also receiving the incoluation.
While these vaccine are going to be enough to immunize 50,000 individuals in the country, as the vaccine requires two doses to be administered to each individual to be most effective, the roughly 500,000 populated country has secured other means of bringing in more vaccines as well.
As per the Health Emergency Operation Center (HEOC), an agreement has been signed with AstraZeneca Pharmaceuticals, to supply Maldives with an additional 700,000 doses of the vaccine. Apart from this, Maldives has also participated in the World Health Organization (WHO) endorsed COVAX facility, which would supply vaccines worth 20 percent of the country’s population. The Minister of Health of Maldives, Ahmed Naseem revealed today that the vaccine shipments to arrive via WHO are expected to be received within the next two weeks, while the 700,000 doses are likely to arrive somewhere during March. Further, an additional 5,000 doses of the Chinese made Sinopharm vaccine has also been donated to the Maldives today.
How safe is it?
Before we question how safe this vaccine is, it is important for us to know what the Covishield vaccine actually is. The Covishield vaccine is produced in Pune of India, by the Serum Institute of India, which has been licensed to mass produce the vaccine by the original producer, AstraZeneca Pharmaceuticals – a British Swedish company, which is globally known for focusing on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas – Oncology, Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide.
In short, the Covishield vaccine is essentially the same as the AstraZeneca vaccine, as the Serum Institute of India is licensed to produce it. The difference of the two vaccines is merely symbolic, given that the same vaccine supplied by AstraZeneca would be known as the Vaccine AstraZeneca, while the one produced by Serum Institute of India would be known as the Covishield vaccine. The collaboration between AstraZeneca and Serum Institute of India has been published on the AstraZeneca website, reassuring us, that the Covishield vaccine given to us is authorized by AstraZeneca.
Briefly looking at how the vaccine works, according to AstraZeneca, the vaccine “uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body.” The vaccine comes in two doses, with the second vaccine required to be administered with in four to six weeks of the initial jab.
As for safety, the Covishield vaccine, according to the Covid-19 vaccine tracker, the Serum Institute of India’s Covishield vaccine is approved in 8 countries so far, including Bahrain, Bangladesh, India, Maldives, Morocco, Nepal, South Africa and in Sri Lanka. Apart from this, the AztraZeneca vaccine is approved in at least 44 different countries, including the UK, France, Germany, Greece, India, etc.
The vaccine also comes approved for use by the European Medicines Agency (EMA), as well as the Maldives Food and Drug Authority (MFDA). Further, COVAX, a World Health Organization (WHO) co-led facility, has also signed an agreement with AstraZeneca, to supply 50 million doses of the vaccine to the participants of the COVAX facility.
COVAX also anticipates that, via an existing agreement with AstraZeneca, at least 50 million further doses of the AstraZeneca/Oxford vaccine will be available for delivery to COVAX participants in Q1 2021, pending emergency use listing by WHO of the COVAX-specific manufacturing network for these doses. A decision on this candidate is also anticipated by WHO in February.
World Health Organization (WHO)
How do I sign up?
The Maldives has made it very easy for anyone living the in the country to receive the Covid-19 vaccine. As per current requirements, anyone resident who wishes to receive the inoculation would be eligible to apply for it, via an online platform.
Signing up to my.health.mv, which is the dedicated platform to sign up for vaccination has been set up fairly easy for anyone who has an internet connection and access to an email or mobile phone, as it required the individual’s basic information, which is then required to be verified via mobile or email.
The portal also allows any individual to be able to assist others who are unable to register themselves to apply for the vaccine, via the option to add dependents.
When will I get the vaccine?
According to the latest announcements by the government of Maldives, vaccination will go on from 1st February until at least six more months, with the aim to vaccinate the entire population of the country within this duration.
During this time, vaccination of those who are more vulnerable to the infection would be prioritized, with front line health care workers at the top of the list, followed by those who fall under the high risk category, such as those who are immunocompromised, or are taking long term medications.
Regardless, the vaccine is within our reach, and will hopefully soon make Maldives one of the first countries to have the entire population vaccinated, according to the Minister of Health of Maldives, Ahmed Naseem.
Until then, the onus is on us to ensure that we remain safe, by exercising the expert recommended health and safety precautions against the virus, such as wearing masks at all times, maintaining a minimum of 6 feet in physical distance and practicing hand and general hygiene as often as possible.